13
SEC
Mobile phase
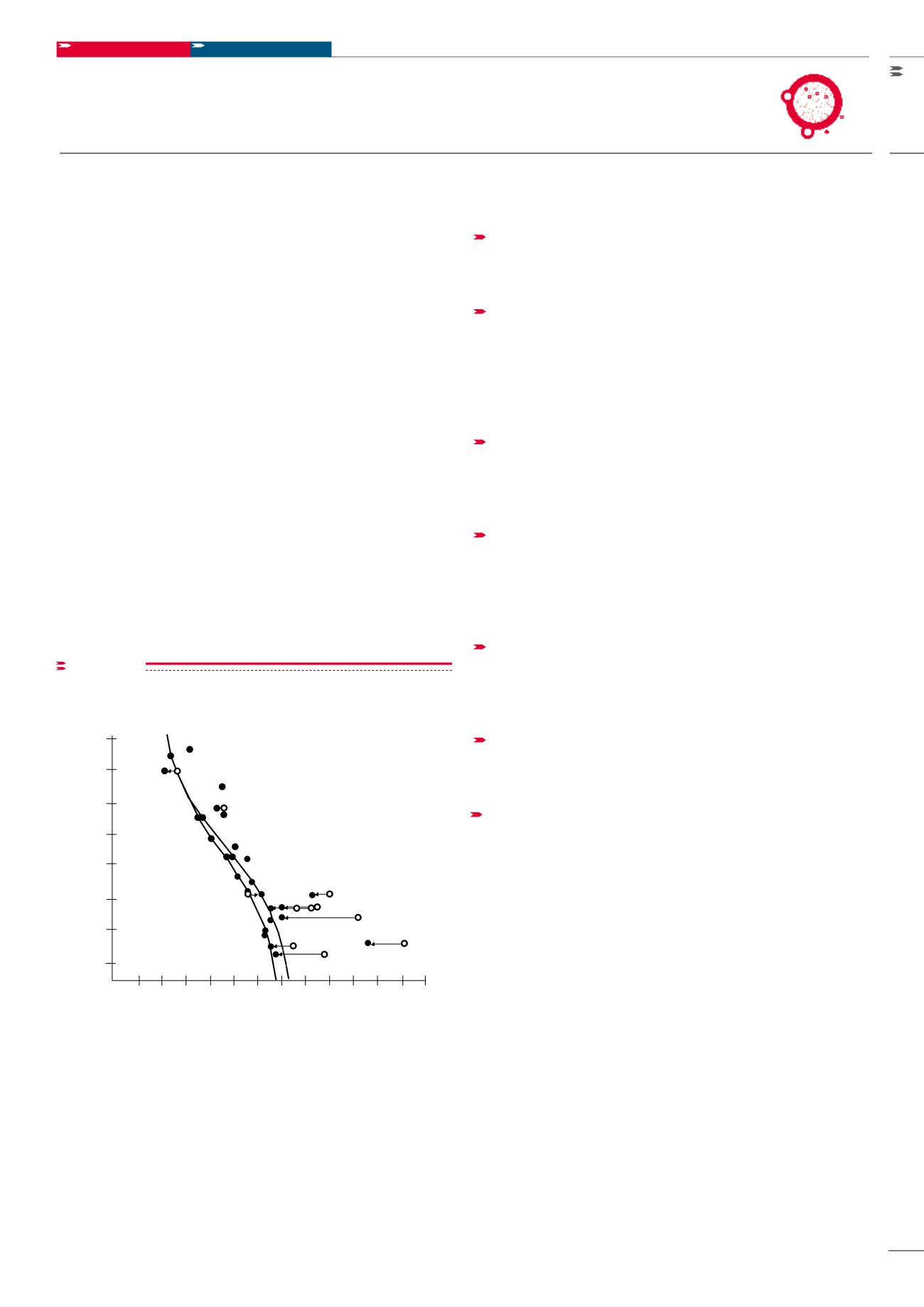
Mobile phase components, such as salts, can affect SEC
separations. The presence or absence of sodium chloride
influences the elution volume of proteins. This is demon-
strated in Figure 4, in which a mixture of various proteins
was separated on a column packed with TOYOPEARL
HW-55F. Salt concentrations can change the hydrody-
namic radius of proteins and either increase or decrease
their molecular size as a function of salt strength. Ideally, in
SEC sample components do not interact with the packing
material. In practice it is often necessary to select a salt
concentration which minimizes secondary interactions
of the sample components with the resin. However, there
are instances where secondary interactions, particularly
hydrophobic interactions at higher salt concentrations, can
be exploited.
It is important to note that relatively minor changes
in protein
structure may affect protein solubility and
encourage secondary
hydrophobic interactions causing
similarly sized proteins or analogs to elute at different
times. In those cases it may be necessary to modify the
mobile phase composition to regain a separation based
on molecular size alone.
Properties of TOYOPEARL SEC resins in aqueous
eluents
High mechanical stability
TOYOPEARL resins can be operated at pressures up
to 3 bar without deformation.
Minimum change in gel bed volume
Changes in the column bed volume under operational
salt conditions are negligible. TOYOPEARL does not
shrink or swell even in high concentrations of strong
denaturing agents such as urea or guanidine
hydrochloride.
chemical stability
TOYOPEARL is stable from pH 2-13, and tolerant to
pH 0-14 for short periods. Biomolecules which are only
soluble at extreme pH values can be readily separated.
sharp chromatographic peaks
TOYOPEARL’s narrow particle size distribution (min. 80%
within declared limits) results in better peak shapes and
higher elution target concentrations than other SEC
materials.
temperature stability
TOYOPEARL is thermally stable and does not degrade or
denature even in boiling water. TOYOPEARL resins can
be sterilized
by autoclaving at 121 °C.
microorganism resistance
TOYOPEARL is an organosynthetic material, and is
resistant to degradation by microorganisms.
suitability for enzyme immobilization
TOYOPEARLresinscontainnumeroushydroxylgroupson
the external and internal bead surfaces. These, in
combination with the chemical stability of the polymer,
make the resin well suited for the covalent bonding of
enzymes or other ligands. (Please see the AFC section for
more information.)
size exclusion
chromatography
5.0
4.5
Log MW
Elution Volume (mL)
Column:
Toyopearl HW-55F, 22mm ID x 50cm
Elution:
25mmol/L Tris-HCl with ( ) or without ( )
0.5mol/L NaCl, (pH 7.5)
Flow rate:
16cm/h
Temperature: 5-10°C
Detection:
UV @ 280nm, 420nm for heme proteins,
200nm for proteins without aromatic amino acid
4.0
5.5
60
100
120
160
140
180
80
Apotransferrin (pl 5.5)
Hemoglobin (pl 6.8 - 7.0)
Yeast ADH (pl 5.4)
Catalase (pl 5.5)
γ
-Globulin (pl 6.6)
Urease (pl 4.8 - 5.1)
Porcine Pepsin (pl ~1.0)
Subtilisin Carlsberg (pl 9.4)
Subtilisin BPN (pl 9.1)
Thermolysin (pl 5.1)
Chymotrypsinogen A (pl 9.5)
Trypsin (pl 10.5)
Lysozyme (pl 11.0)
RNase A (pl 9.5)
Cytochrome C (pl 10.6)
Comparison of the elution volumes of proteins in
presence and absence of NaCl
Column: TOYOPEARL HW-55F, 22 mm ID x 50 cm L; Elution: 25 mmol/L
Tris-HCl with (
•
) or without (
o
) 0.5 mol/L NaCl, (pH 7.5); Flow rate: 16 cm/h;
Temperature: 5 - 10 °C; Detection: UV @ 280 nm, 420 nm for heme proteins,
200 nm for proteins without aromatic amino acid
figure 4
c
of the eluti n volumes of proteins in
presence and absence of nacl


