AFC
AFC
95
figure 1
TSKgel affinity chromatography column packings
CH
2
OSO
2
CH
2
CF
3
TSKgel Boronate-5PW
TSKgel Tresyl-5PW
B OH
HO
TSKgel Chelate-5PW
CH
2
COOH
CH
2
COOH
TSK-GEL affinity chromatography column packings
O–R
O–R
O–R
G5000PW
G5000PW
G5000PW
CH
2
OSO
2
CH
2
CF
3
TSKgel Boronate-5PW
TSKgel Tresyl-5PW
B OH
HO
TSKgel Chelate-5PW
CH
2
COOH
CH
2
COOH
TSK-GEL affinity chromatography column packings
O–R
O–R
O–R
G5000PW
G5000PW
G5000PW
CH
2
OSO
2
CH
2
CF
3
TSKgel Boronate-5PW
TSKgel Tresyl-5PW
B OH
HO
TSKgel Chelate-5PW
CH
2
COOH
CH
2
COOH
O–R
O–R
O–R
G5000PW
G5000PW
G5000PW
Applications of TSKgel affinity chromatography columns
Separation columns should be protected with a guard column. Tosoh
Bioscience offers a unique Guardgel kit consisting of guard column
hardware and gel packing, allowing the user to repack the guard column
as required. Guardgel kits are available for most affinity columns, both
glass and stainless steel.
TSKgel BORONATE-5PW
Coupling of m-aminophenyl boronate to the TSKgel 5PW-type polymeric
support results in a ligand capable of forming a tetrahedral boronate
anion under alkaline pH conditions. This anionic structure can bind with
1,2 cis-diol groups such as those found in carbohydrates, carbohydrate-
containing compounds, and catecholamines. Interaction between the
boronate anion and the 1,2 cis-diol groups is enhanced in the presence
of Mg
2+
ions and is inhibited by amine-containing buffers. Adsorption
onto the TSKgel Boronate-5PW takes place in basic buffers such as
HEPES and morpholine, while desorption takes place in carbohydrate
or amine-containing mobile phases like sorbitol or Tris.
Applications for TSKgel Boronate-5PW include: nucleic acids,
nucleotides and nucleosides. This affinity column has also been used to
isolate catecholamines and other biomolecules containing the 1,2 cis-
diol functionality (
Figure 2
).
TSKgel CHELATE-5PW
TSKgel Chelate-5PW utilizes the ability of iminodiacetic acid (IDA)
to chelate ions such as Zn
2+
, Ni
2+
and Cu
2+
. The column is pre-loaded
with divalent metal ions by chelation. Peptides and proteins containing
histidine residues will normally adsorb to these chelated ions at neutral
pH. The retained compounds are then eluted with buffer containing
imidazole or glycine.
The key to making successful use of this retention mechanism is the
proper selection of metal ions for chelation and the elution buffer to
desorb the analytes. In general, Cu
2+
interacts better with protein;
however, resolution is usually enhanced with Zn
2+
ions. A gradient
mobile phase containing increasing imidazole or glycine concentrations
is used to elute the retained compounds. A decreasing pH gradient
can also be used. Glycine, as well as HEPES buffers, will also elute
the metallic ion so column regeneration is necessary. Conversely,
imidazole in phosphate buffer will extract the metal ions very slowly,
avoiding frequent column regeneration.
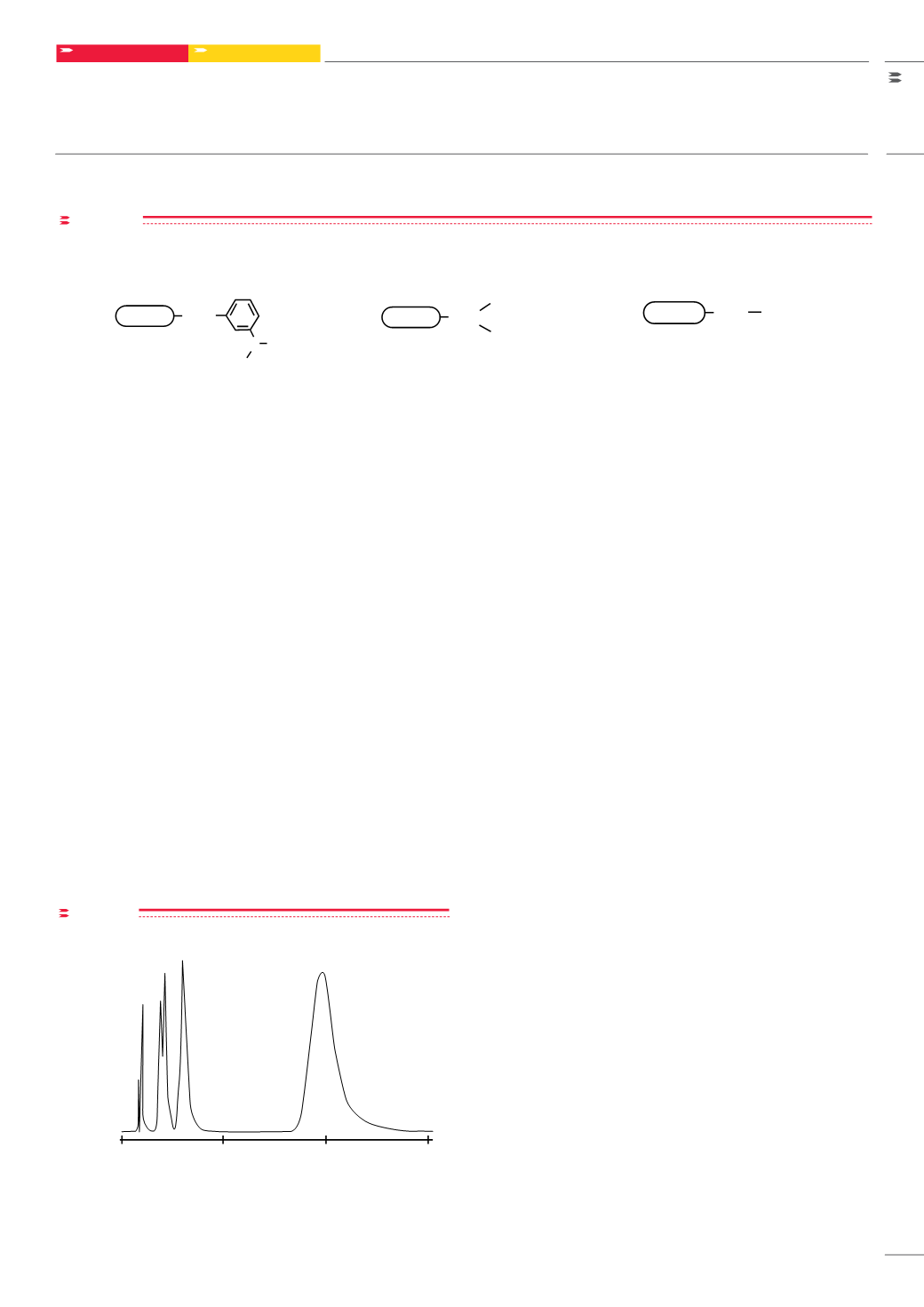
Figure 2
Separation of catecholamines on TSKgel Boronate-5PW
Separation of catecholamines on TSKgel Boronate-5PW
Column:
Sample:
Elution:
Flow Rate:
TSKgel Boronate-5PW, 7.5mm ID x 7.5cm
1. tyrosine, 2. normetanephrine, 3. metanephrine,
4. DOPA, 5. epinephrine
0.1mol/L phosphate buffer, pH 6.5
1.0mL/min
Minutes
0
15
30
45
1 2
3 4
5
Column: TSKgel Boronate-5PW, 7.5 mm ID x 7.5 cm L; Sample: 1. tyrosine,
2. normetanephrine, 3. metanephrine, 4. DOPA, 5. epinephrine;
Elution: 0.1 mol/L phosphate buffer, pH 6.5; Flow rate: 1.0 mL/min;
Detection: UV @ 280 nm


