96
AFC
Applications for TSKgel Chelate-5PW include: immunoglobulins,
transferrin, lectins, milk proteins, membrane proteins, and peptides.
In
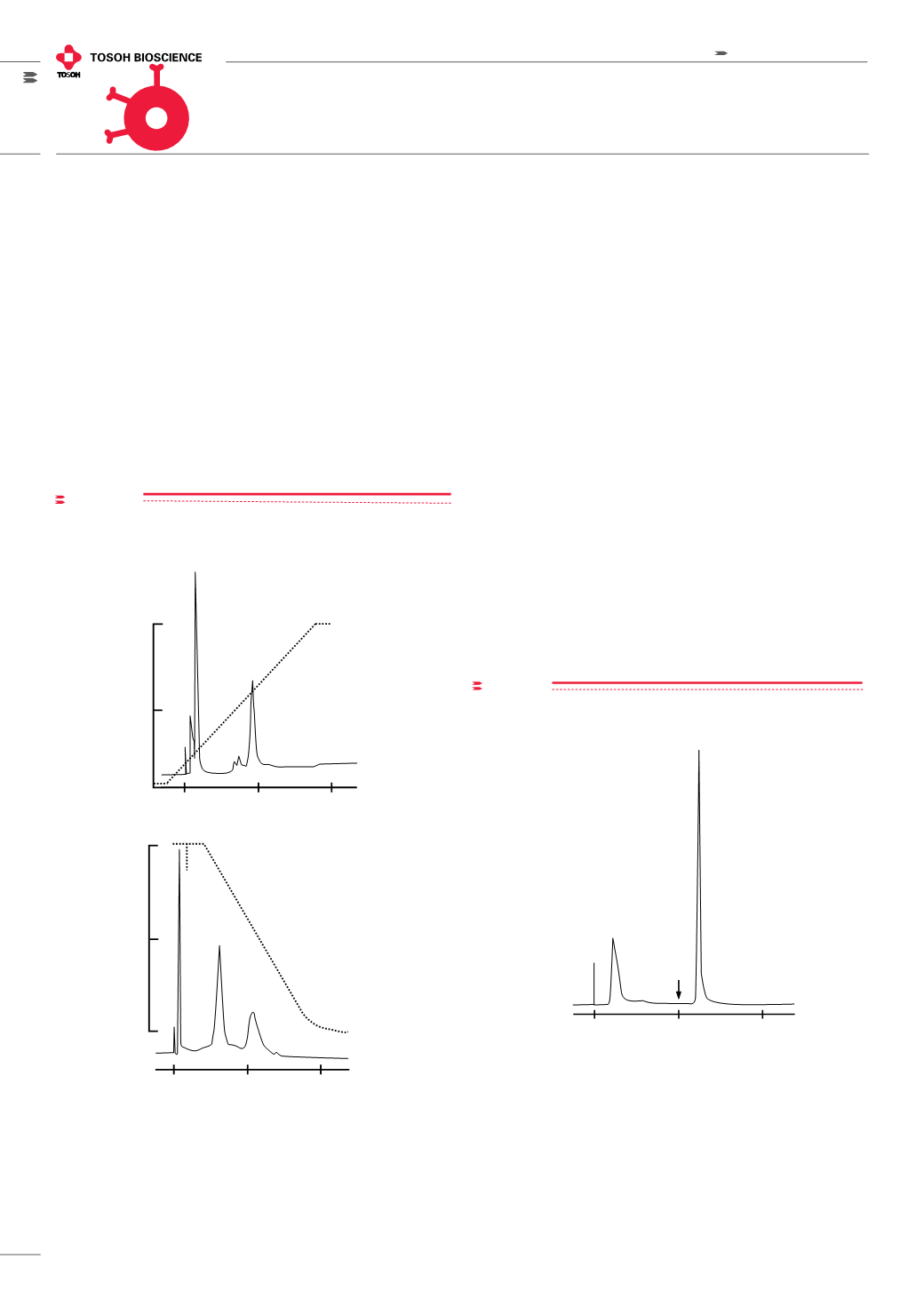
Figure 3,
the separation of ribonuclease A (bovine) and transferrin
(human) are compared on TSKgel Chelate-5PW columns (glass,
5 mm ID x 5 cm L) containing different metal ions.
TSKgel TRESYL-5PW
Unlike other TSKgel affinity columns, the TSKgel Tresyl-5PW (tresyl;
2,2,2-trifluoroethanesulfonyl) requires activation with a user-selected
ligand containing amino, thiol, phenol, or imidazole groups. The resulting
structure is literally a custom affinity ligand with excellent pH stability
and minimal ligand loss due to leaching. TSKgel Tresyl-5PW readily
reacts with amino or thiol groups to form stable covalent alkylamines
or thioethers.
FIgure 4
Purification of peroxidase on concanavalin A coupled to
TSKgel Tresyl-5PW
Washing step:
Ligand solution:
Coupling step:
Blocking step:
Column:
Sample:
Binding:
Elution:
Flow Rate:
Detection:
Wash TSKgel Tresyl-5PW, 6mmID x 4cm, with DI water
Dissolve 40mg of concanavalin A in 10mL of
0.1mol/L NaHCO
3
, pH 8.0, containing 0.5mol/L NaCl
Recycle the ligand solution overnight through the
column at 0.2mL/min at 25°C
Block residual tresyl groups with 0.1mol/L Tris-HCl,
pH 8.0, at 1.0mL/min for 1hr at 25°C
TSKgel Tresyl-5PW modified with concanavalin A
Crude peroxidase, 0.5mg
0.05mol/L acetate buffer, pH 5.0, containing 0.5mol/L
NaCl and 1mmol/L each of CaCl
2
, MnCl
2
, and MgCl
2
Step gradient at 4min (see arrow on diagram)
to 25mmol/L
a
-methyl-D-glucoside in binding buffer
1.0mL/min
UV @ 403nm
Minutes
0
4
8
Washing step: Wash TSKgel Tresyl-5PW, 6 mm ID x 4 cm L, with DI water;
Ligand solution: Dissolve 40 mg of concanavalin A in 10 mL of 0.1 mol/L
NaHCO
3
, pH 8.0, containing 0.5 mol/L NaCl; Coupling step: Recycle the
ligand solution overnight through the column at 0.2 mL/min at 25°C;
Blocking step: Block residual tresyl groups with 0.1 mol/L Tris-HCl, pH 8.0, at
1.0 mL/min for 1 h at 25°C; Column: TSKgel Tresyl-5PWmodified with concana-
valin A; Sample: Crude peroxidase, 0.5 mg; Binding: 0.05 mol/L acetate buffer,
pH 5.0, containing 0.5mol/L NaCl and 1mmol/L each of CaCl
2
, MnCl
2
, andMgCl
2
;
Elution: Step gradient at 4 min (see arrow on diagram) to 25 mmol/L -methyl-
D-glucoside in binding buffer; Flow rate: 1.0mL/min; Detection: UV @ 403 nm
Applications of TSKgel affinity chromatography columns
figure 3
Separation of standard proteins by immobilized metal ion affinity chro-
matography
proteins by immobilized metal ion affinity chromatography
1
B. Zn
2+
C. Ni
2+
2
1
30
15
0
8
6
4
pH
Minutes
Minutes
tes
0
15
30
2
30
20mM
0mM
te-5PW, 5mm ID x 5cm
n
2+
, and C. Ni
2+
se A (bovine), 2. transferrin (human)
in linear gradient from 1mmol/L to 20mmol/L imidazole in 20mmol/L HEPES-NaOH buffer, pH 8.0, containing 0.5mol/L NaCl
r pH gradient from 20mmol/L HEPES-MES-acetic acid, pH 8.0, to 20mmol/L HEPES-MES-acetic acid, pH 4.0, both in
l
A) Zn
2+
hromatography
C. Ni
2+
2
1
30
15
0
8
6
4
pH
s
Minutes
30
azole in 20mmol/L HEPES-NaOH buffer, pH 8.0, containing 0.5mol/L NaCl
acid, pH 8.0, to 20mmol/L HEPES-MES-acetic acid, pH 4.0, both in
B) Ni
2+
Column: TSKgel Chelate-5PW, 5 mm ID x 5 cm L; Metal Ion: A) Zn
2+
and B) Ni
2+
Sample: 1. ribonuclease A (bovine), 2. transferrin (human)
Elution: A): 30 min linear gradient from 1 mmol/L to 20 mmol/L imidazole in
20 mmol/L HEPES-NaOH buffer, pH 8.0, containing 0.5 mol/L NaCl
B) 30 min linear pH gradient from 20 mmol/L HEPES-MES-acetic acid, pH
8.0, to 20 mmol/L HEPES-MES-acetic acid, pH 4.0, both in 0.5 mol/L NaCl;
Flow rate: 0.8 mL/min; Detection: UV @ 280 nm


