56
AFC
TOYOPEARL Resins for Protein A Affinity Chroma-
tography
Protein A affinity chromatography is the most commonly
used capture step in antibody purification processes. Its
high specificity for the binding of human immunoglobulin
allows highly selective capturing of the target protein out
of cell culture supernatant. The protein A capture step is
most often followed by ion exchange, HIC or mixed-mode
polishing steps in order to remove nucleic acids, aggre-
gates and leached protein A.
The first protein A affinity resins were introduced in the
1970s based on native protein A ligands derived from the
bacterium Staphylococcus aureus. These media suffered
from insufficient alkaline stability, which limited the
cleaning in place options for process use. State-of-the-art
protein A resins carry recombinant protein A variants
genetically engineered to provide maximum IgG affinity
and base stability.
Tosoh Bioscience offers two protein A affinity resins, both
based on alkaline stable, recombinant ligands coupled to
the proven TOYOPEARL polymethacrylate matrix. The new
ultra-high capacity TOYOPEARL AF-rProtein A HC-650M
excels all other commercially available protein A media
with regard to its IgG binding capacity.
Protein A Chromatography – How does it work
Protein A is a 40-60 kDa surface protein originally found in
the cell wall of the bacteria Staphylococcus aureus. Protein
A and its recombinant derivatives bind the Fc region of
immunoglobulins through interaction with the heavy chain.
The binding strength of protein A for IgG depends on
the source species of the immunoglobulin as well as the
subclass of IgG. The standard protocol for antibody purifi-
cation by protein A chromatography involves loading of the
feedstock at physiological pH and ionic strength, washing
unbound substances of the column with loading buffer and
elution of the bound immunoglobulins by lowering the pH.
The change in pH alters the degree of ionization of charged
groups on the ligand and the bound antibody thus reducing
the affinity. The fractions can be collected into neutraliza-
tion buffer to return to a neutral pH.
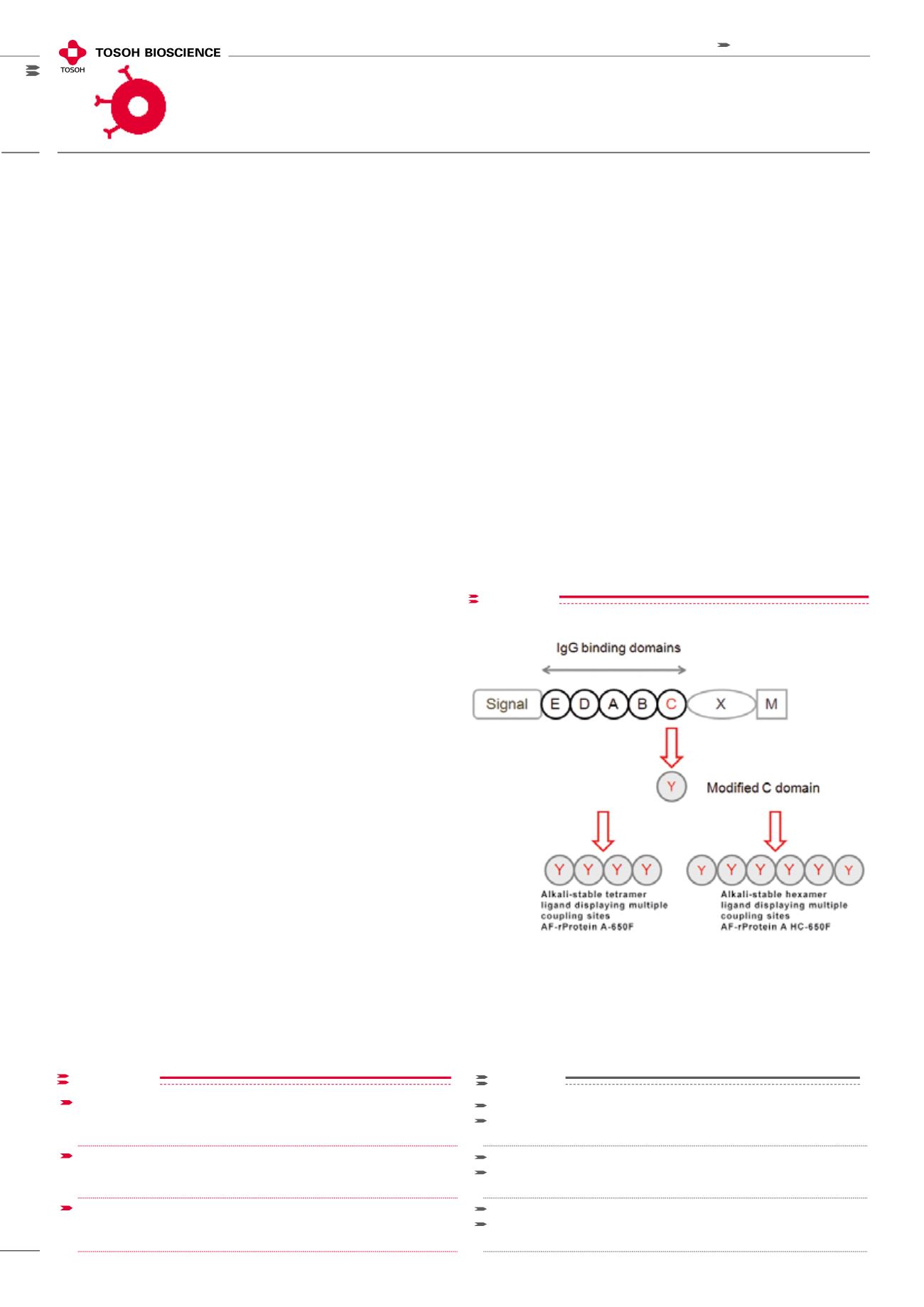
TOYOPEARL Protein A RESINS
The ligands of all TOYOPEARL protein A resins are recombi-
nant protein A variants expressed in E. coli. They are derived
from one of the IgG binding domains of protein A. The
amino acid sequence is optimized in order to increase the
protein’s stability towards alkaline solutions and to intro-
duce additional lysine residues that can be utilized for multi-
point attachment of the ligand to the TOYOPEARL matrix.
The ligand of TOYOPEARL AF-rProtein A-650F consists of
a tetramer of these modified protein A C domains. For the
ultra-high capacity TOYOPEARL AF-rProtein A HC-650F this
domain was further optimized and expressed as a hexamer
in order to further increase IgG binding capacity (Figure 1).
Multipoint attachment of the ligand to the TOYOPEARL
matrix enhances the chemical and thermal stability of the
resin. In practice this pays off for a low level of protein A
leaching and also for a high resistance to alkaline solutions.
Both resins are based on the TOYOPEARL HW-65F base
bead with a particle size of 45 μm.
Protein a Affinity
chromatography
features
Benefits
High IgG binding capacity
Increased productivity of antibody purification
Lower buffer consumption
Recombinant protein A ligand
Alkaline stable
Low protein A leakage
TOYOPEARL polymer matrix
High mechanical stability
High chemical stability
figure 1
RECOMBINANT PROTEIN A DERIVED LIGANDS


