TOSOH
CUSTOMER MAGAZINE
The dynamic binding capacity (DBC) of a stationary phase is influ-
enced by the contact time between the sample and the ligand, the so-
called residence time. Good mass transfer properties enable a resin
to reach a high binding capacity even at high flow rates. The capacity
of the new resin was tested at various residence times and mAb ti-
ters. Figure 1a & b show the breakthrough curves for TOYOPEARL
AF-rProtein A HC-650F at two feed concentrations. The resin shows
complete mAb adsorption until breakthrough occurs. This remains
unaffected even for short residence times of 1 min. The measured
capacities of more than 100 mg/mL exceed the DBCs of all other base
stable Protein A resins.
To further evaluate the elution properties, a purified humanized mo-
noclonal IgG was diluted to a final concentration of 4.75 g/L. Simula-
ting high HCP density in the feed, aliquots were spiked with concen-
trated cell culture fluid. Protein A chromatography with TOYOPEARL
AF-rProteinA HC-650F was conducted 200 µL RoboColumns using a
robotic chromatography station. The total loaded mass was varied
from 10 to 50 mg/mL resin. A residence time of 2 minutes was ap-
plied. Before elution, the columns were washed with 20 column vo-
lumes of loading buffer.
MAb elution with acetate buffer, pH 3.25 delivered more than 95 %
mAb recovery. Due to the acidic pH applied for elution, mAbs are
prone to aggregation. Naturally, high capacity Protein A resins ad-
sorb large amounts of mAb. This might enhance mAb aggregation
due to higher protein concentrations in the elution pool. Thus, special
attention was paid to the aggregate content after elution of the bound
antibody.
Size exclusion chromatograms of two mAb elution pools are shown
in Figure 2. The elution pools of 10 mg/mL and 50 mg/mL mAb load
were injected, respectively. Although the SEC chromatograms seem
to show aggregates for the higher loading only, a closer look reveals
similar aggregate contents when referring to the corresponding total
protein amount. Both pools contain 0.6 % aggregates.
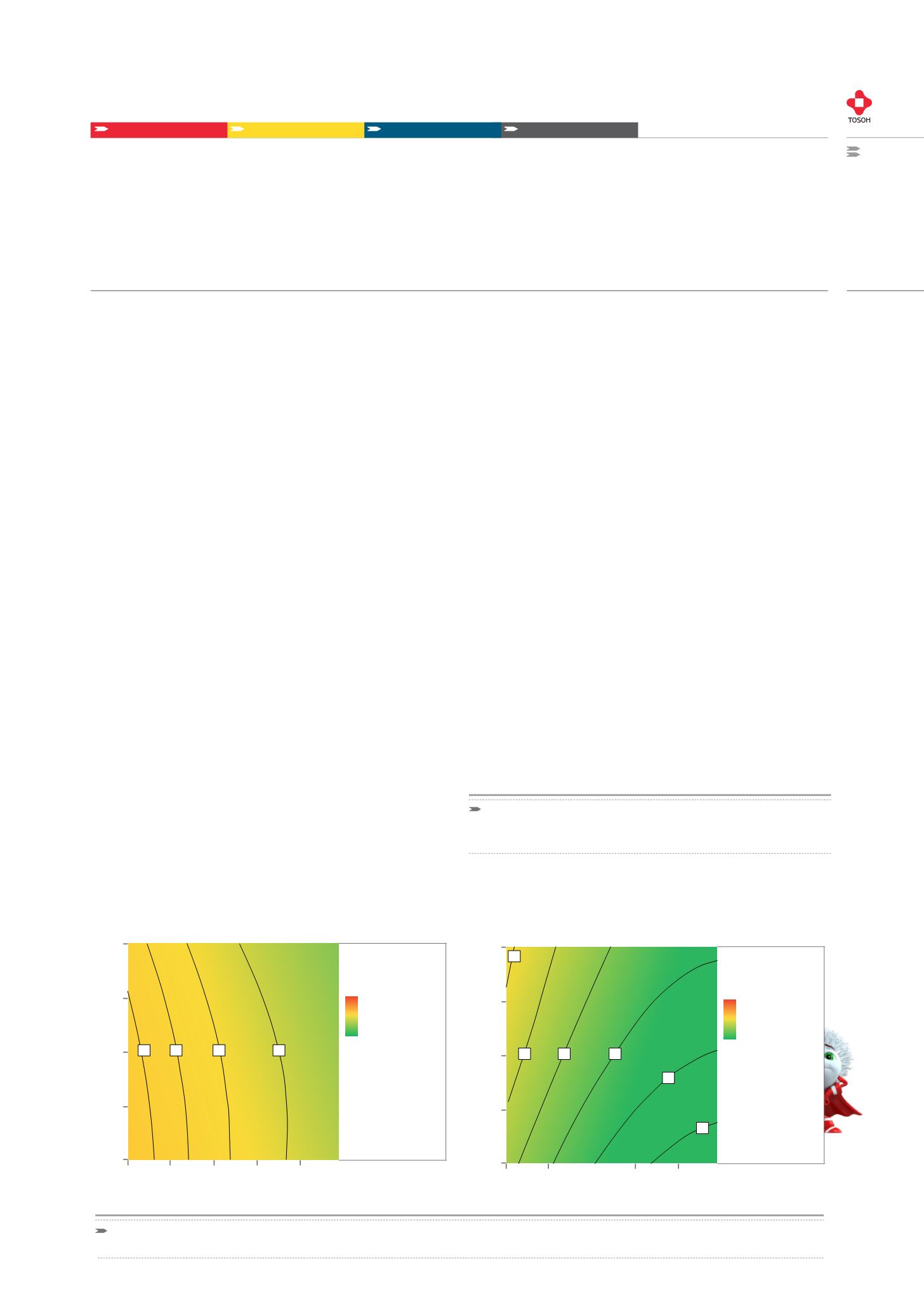
In biopharmaceutical manufacturing, low Protein A ligand leaching
is crucial and needs to be proved by ELISA testing. Protein A lea-
ching was analyzed for 2.5 g/L and 7 g/L concentrated feed streams.
Spiking and residence time were kept constant. Figure 3 shows that
the absolute load has little influence on numeric Protein A leaching.
Overall, Protein A leaching does not exceed 45 ppm for any of the
tested pH and load conditions. Higher absolute mAb loadings seem
to be advantageous, since the relative Protein A content of the mAb
pool decreases.
Considering the obtained results regarding Protein A leaching, ag-
gregate content and protein adsorption, high titers seem favorable
for Protein A chromatography. This mAb seemed unaffected with re-
gards to aggregation, and was efficiently adsorbed, which reduces
Protein A cycle time. Further, Protein A leaching was even lower when
applying higher titers. Thus, ultra-high capacity Protein A resins offer
additional benefits besides reducing costs because less resin volume
is needed to purify a given amount of monoclonal.
AUTHORS
JUDITH VAJDA, TOSOH BIOSCIENCE GmbH, ANGELIKA WACKER, UNIVERSITY OF
APPLIED SCIENCES MANNHEIM
2.75
2.95
3.15
3.35
3.55
elution pH
30.00
25.00
20.00
35.00
40.00
ProteinA leaching (ng/mL)
B: load
Design-Expert®Software
Factor Co
ProteinA leaching
4
5
6
7
X1= A:ph
X2= B:load
Actual Factors
C: titer = 2.50
D: spiking = 15.00
20
0
2.75
2.95
3.15
3.35
3.55
elution pH
30.00
25.00
20.00
35.00
40.00
ProteinA leaching (ng/mL)
B: load
Design-Expert®Software
Factor Co
ProteinA leaching
X1= A:ph
X2= B:load
Actual Factors
C: titer = 7.00
D: spiking = 15.00
20
0
5
3
4
2
1
0
FIGURE 3: PROTEIN A LEACHING
Contour plots for TWO different load concentrations. Protein A leaching is plotted against pH and absolute load. A: 2.5 g/L. B: 7 g/L.


