44
HIC
TOYOPEARL resins for Hydrophobic Interaction
Chromatography
Hydrophobic interaction chromatography (HIC) is a power-
ful tool for the process purification of biomolecules. The
technique utilizes the accessible hydrophobic regions
located on protein surfaces and their interactions with a
weakly hydrophobic stationary phase. HIC is an excellent
complement to ion exchange (IEC) and size exclusion chro-
matography (SEC) particularly when protein isoforms exist
or when feedstock impurities are of similar isoelectric point
or molecular weight. The selectivity differences exploited
by HIC can also be used after affinity separations in which
closely related proteins with similar recognition sites are
not distinguishable by the affinity ligand.
How does HIC work?
Proteins and other molecules with hydrophobic surfaces
are attracted to the hydrophobic ligands of both reversed
phase (RPC) and HIC resins. RPC resins have higher surface
coverage and/or more hydrophobic
ligand compared to
HIC resins. Because of this, in a RPC separation the
target
binding readily occurs in an aqueous solution, and desorp-
tion is
promoted by the addition of an increasing amount
of organic solvent.
In HIC, proteins are bound to the resin by employing
an aqueous high salt mobile phase. The salt conditions
contribute to a lyotropic effect which allows the proteins to
bind to the lower surface coverage of a hydrophobic ligand.
Proteins are eluted by the simple technique of decreasing
the salt concentration. Most therapeutic targets are eluted
in a low salt or a no salt buffer.
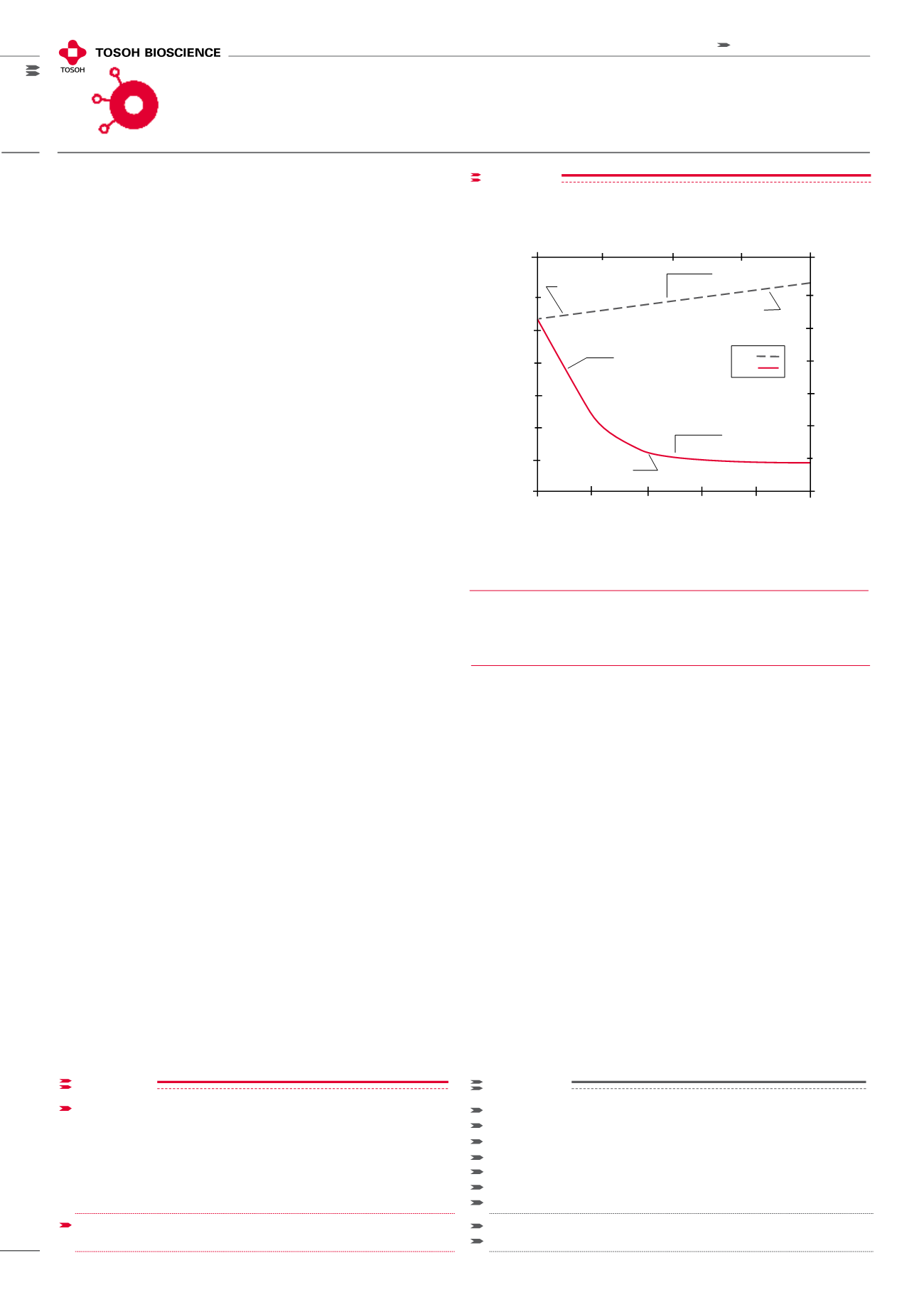
During elution the energy of interaction for a HIC step is less
than that of a RP step. One means of gauging the relative
binding energy between the two techniques is to measure
the surface tension of the two sets of binding and elution
conditions. Figure 1 provides a comparison of the surface
tension generated by HIC and RPC elution systems. Since
HIC separates under milder eluting conditions, biological
activity is typically retained.
hydrophobic interaction
chromatography
figure 1
Mode Gradient (Typical)
∆
Surface Tension
(erg/cm
2
)
HIC 1.8 to 0mol/L 4
(NH
4
)
2
SO
4
/ aqueous buffer
RPC 10 to 50% ACN/ 0.1%TFA 23
C. Horvath et. al., Separation Processes in Biotechnology, (J. Asenjo, Ed.)
9, 447 (1990) Marcel Dekker
The surface tension of aqueous solutions used
in HIC and RPC
Surface Tension (erg/cm
2
)
(NH
4
)
2
SO
4
Concentration (mol/L)
% Acetonitrile
HIC
RPC
0 1 2 3 4
0 20 40 60 80 100
80
60
40
20
Desorb protein
Desorb protein
Bind protein
Bind protein
(NH
4
)
2
SO
4
CH
3
CN
Mode Gradient (typical)
∆
Surface tension
(erg/cm
2
)
HIC 1.8 to 0 mol/L
4
(NH
4
)
2
SO
4
/ aqueous buffer
RPC 10 to 50 % ACN/ 0.1 % TFA
23
C. Horvath et. al., Separation Processes in Biotechnology, (J. Asenjo, Ed.)
9, 447 (1990) Marcel Dekker
features
Benefits
hydrophilic polymer resin matrix
robust chemical stability between pH 1 - 13
temperature range 4 - 60 °C
autoclavable at 121 °C
compatible with organic solvents
constant bed volume over a wide range of salt concentrations
low non specific protein binding
superior protein recovery
good mechanical stability
excellent flow characteristics in large industrial size columns
direct scale-up from TSKgel HIC HPLC columns
surfac tension of aqueous s luti ns u ed in HIC & RPC


