FAQs on Analysis with Size Exclusion Chromatography
Which protein standard can be used to test my SEC system?
A protein standard for size exclusion chromatography contains proteins of different molecular weights and is used to either calibrate the system or to determine how good the separation works. To this end, the number of theoretical plates is calculated for a specific protein in the mixture. The standard formulas for the number of theoretical plates differ in the US, EU, and Japan. Furthermore, different protein mixtures result in different plate numbers due to a different composition. These two reference standards are often used:
- Merck/Sigma SEC Protein Standard mixture 15 – 600 kDa (#69385)
- BioRad Protein Standard (TP calculation based on VitB12 is around 10% lower compare to pABA)
What is the difference between “SW” and “PW” SEC columns?
The “S” in SW, SWXL and UP-SW stands for silica so these columns are created using porous silica. The “P” in PW and PWXL refers to a polymeric support of porous methacrylate. The “W” for both column lines refers to water and indicates the use with aqueous mobile phases.
What causes tailing in size exclusion chromatography?
The TSKgel SW/SWXL columns contain porous silica that has been chemically bonded with proprietary hydrophilic material. This coating stabilizes the silica in aqueous solvents. When charged, strongly polar, or hydrophobic molecules interact with the silica support, it is possible to have secondary electrostatic, hydrophilic or hydrophobic interaction between the solutes and the silica matrix. Thus, the separation involves two mechanisms, size exclusion and electrostatic, hydrophilic or hydrophobic interaction. When this occurs the chromatogram may exhibit peak tailing, as the number of adsorption sites that cause the secondary interaction is limited. The best way to avoid secondary interaction is to use a buffer with an ionic strength in excess of 200 mM, e.g. 100 mM phosphate buffer and 100 mM Na2SO4, pH 6.8.
How much sample can I load onto an SEC column?
The amount of sample that can be loaded onto an SEC column depends on column dimension (maximum 1 % of the column volume) and on the particle size. Below you find you will find values with which our columns are often used:
- TSKgel SWXL (7.8 mm ID x 30 cm L): 15-150 µl of a 1-10 mg/ml solution
- TSKgel UP-SW (4.6 mm ID x 30 cm L): 1-10 µl 1-10 mg/ml solution
- For analysis: lower volume is better!
Which salt concentration should I use for SEC?
Salt reduces potential interaction with the stationary phase and thus improves the separation by SEC. For the separation of proteins, a 100 mM phosphate buffer with and 100 mM of NaCl or Na2SO4 can be used as a standard. The maximum salt concentration to be used with most of our SEC columns is 0.5 M of NaCl. The maximum of each column is also indicated in the operating conditions and safety sheets.
Can I use a GFC column with organic solvent?
It depends on the column whether or not GFC columns can be used with organic solvents. For example, TSKgel SW columns can be used with up to 100% water-miscible organic solvents, PW type columns can stand up to 20 % and even 50 %, if the solvent change is done with a shallow gradient.
My new SEC column has a low protein recovery – can I avoid this?
There are active sites on the resin that require conditioning/saturation with either your sample protein or another material such as bovine serum albumin (BSA). For our silica-based columns (SW-type) we recommend 4 successive injections of 100 µg BSA to condition the resin. The PW/PWXL sizing columns are far less hydrophilic and normally do not require conditioning
How do I select a size exclusion column?
The choice of a size exclusion column depends on the size of the analyzed molecule, the type of solvent which should be used for separation, whether HPLC or UHPLC should be used and which resolution needs to be achieved. Below suggestions for SEC column collection are given.
The size of analyzed molecules determines the pore size
Separation by SEC is based on different molecule sizes (hydrodynamic volumes) entering the pores of the stationary phase, either better or worse. Therefore, the size of the pores is decisive for the range in which molecules of different sizes are separated. A molecular weight (MW) range is given by vendors to indicate which molecules can be separated on a specific SEC column. The information is often underlined by a calibration curve that plots the retention time of a standard containing molecules with different MWs against the MW to illustrate which range separation is efficient. Care must be taken to ensure that the analyte is structurally similar to the MW standard used for calibration, since the size, which is decisive for the separation, does not necessarily correlate with the MW. If the pore size is to be selected for protein analysis, calibration should have been performed with a protein-based MW standard.
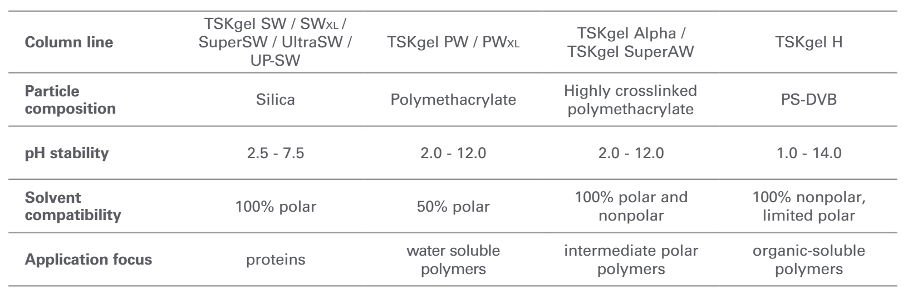
The type of solvent determines the base matrix
The sample that needs to be analyzed often requires a specific solvent. For instance, organic solvents are required for synthetic polymers to be soluble. In contrast, proteins require aqueous buffers around pH 7 to keep it in native conditions and oligonucleotides require a high pH to ensure the absence of secondary structures. This explains the variety of particle materials used for SEC.
Table 1: Particle composition of TSKgel size exclusion columns and their applications
|
 |
The resolution depends on the particle size and column length
Another selection option is the particle size: smaller particles deliver a higher resolution while increasing the backpressure. If very small particles are used (2 µm and smaller), columns are referred to as UHPLC columns mainly used with specific instruments that stand higher pressures and provide very low dead volumes. Which particle size fits the application depends on the sample complexity and how large size differences are in the molecules that need to be separated. A second parameter that influences the resolution is the size of a column: the longer the column – the higher the resolution. In addition to resolution, the column length also impacts throughput with shorter columns providing faster analysis.