FAQs on Analysis with Hydrophobic Interaction Chromatography
How does hydrophobic interaction chromatography (HIC) differ from reversed-phase (RP) chromatography?
Both chromatographic methods employ a hydrophobic stationary phase in order to separate molecules as to their hydrophobicity. However, the stationary phases of HIC columns are often less hydrophobic than those of reversed phases columns. Secondly, elution occurs at different conditions. Whereas HIC employs for binding high salt conditions to increase surface tension and make hydrophobic molecule parts available to interact with the stationary phase, reversed-phase employs a highly polar mobile phase (water) to support interaction of hydrophobic molecules with the stationary phase. This main difference explains why HIC with its non-denaturing conditions is preferably used to analyze proteins. The differences and similarities are shown in the table below.
Table 1:Differences between hydrophobic interaction chromatography (HIC) and reversed-phase chromatography (RP)
|
|
HIC |
RP |
| Mobile phase and gradient |
High salt to low salt |
Low organic to high organic |
| Separation according to |
Hydrophobicity |
Hydrophobicity |
| MS-compatible |
Limited |
yes< |
| Method development |
Many variables require fine-tuning (salt concentration, pH, salt type, flow rate) |
Regarded easier (solvent, start conditions and gradient, more experience) |
| Protein denaturation |
no |
yes |
|
How do I choose salt and salt concentration for HIC separations?
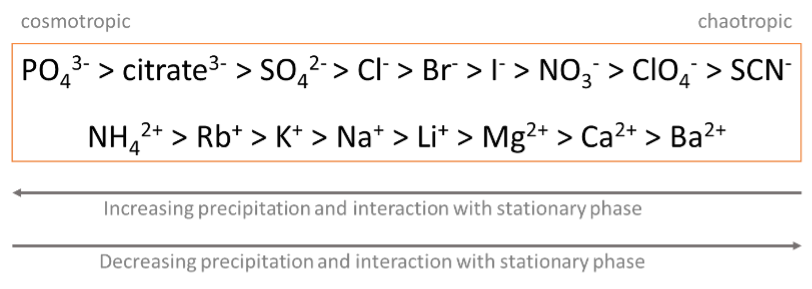
Different salt types have different capabilities to promote the binding of molecules to the stationary phase. While more cosmotropic salts increase the interaction with the stationary phase but also increase the risk of protein precipitation, chaotropic salts are weaker in promoting interaction of molecules with the hydrophobic phase. An order from cosmotropic to chaotropic can be found in the Hofmeister series.
Figure 1: Hofmeister series of cations and anions and the effect on protein precipitation
|
 |
Apart from influencing the binding strength, different salts exhibit different selectivities, so that testing different salts or mixtures of salts can improve the HIC separation.
The salt concentration depends on different aspects:
- Type of salt: cosmotropic salts require a rather low concentration (e.g. 1 M (NH4)2SO4), chaotropic salts require higher concentrations (e.g. 3 M NaCl)
- Hydrophobicity of the stationary phase: Less hydrophobic phases need higher salt concentration than more hydrophobic phases
- Molecule: bigger molecules require lower salt concentrations (e.g. 1 M (NH4)2SO4) for an antibody with 150 kDa) and smaller molecules require higher salt concentrations (e.g. 3 M (NH4)2SO4) for a protein <20 kDa)
Generally, the lowest possible salt concentration should be used.
What is the order of hydrophobicity of TSKgel® HIC columns?
The order of hydrophobicity of TSKgel HIC (U)HPLC columns is depicted below. Please keep in mind, the hydrophobicity of the stationary phase is influenced not only by its functional group but also by other parameters such as ligand density. Therefore, the hydrophobicity of TSKgel HIC HPLC columns does not follow the hydrophobicity of the ligands alone or the hydrophobicity of process resins.
Figure 2: Hydrophobicity of TSKgel HIC columns
|
 |