Tosoh
customer magazine
Oligosaccharides conjugated to carrier proteins (neo-glycoproteins)
have been successfully developed as semi-synthetic vaccines. The con-
cept of using synthetic glycoproteins has also been discussed for the
prevention and therapy of several non-infectious diseases. The com-
plexity of glycosylated proteins necessitates the development of new
analytical strategies to characterize these biopharmaceuticals as their
structure is important for their stability, folding, efficacy, and safety.
Characterization of glycoproteins in terms of identity, heterogeneity
and impurity can be accomplished by a variety of analytical methods:
NMR, MS, CE, HPLC, and spectrophotometric methods. However, li-
quid chromatography (LC) coupled to electrospray ionization mass
spectrometry (ESI-MS) is the most commonly used approach and has
been applied for the analysis of intact glycoproteins, characterization
of glycan structures and glycopeptides. The analysis of the intact pro-
teins is an elegant approach that simplifies sample preparation, but
protein heterogeneity can limit resolution.
The paper focusses on the development of a simple HILIC-UV method
for the analysis of intact glycoproteins to be used for the monitoring of
synthetic glycosylation processes. As proof of concept ribonuclease A
(RNase A) and RNase B which exists in five isoforms varying in the
number of mannose residues were separated to optimize the method.
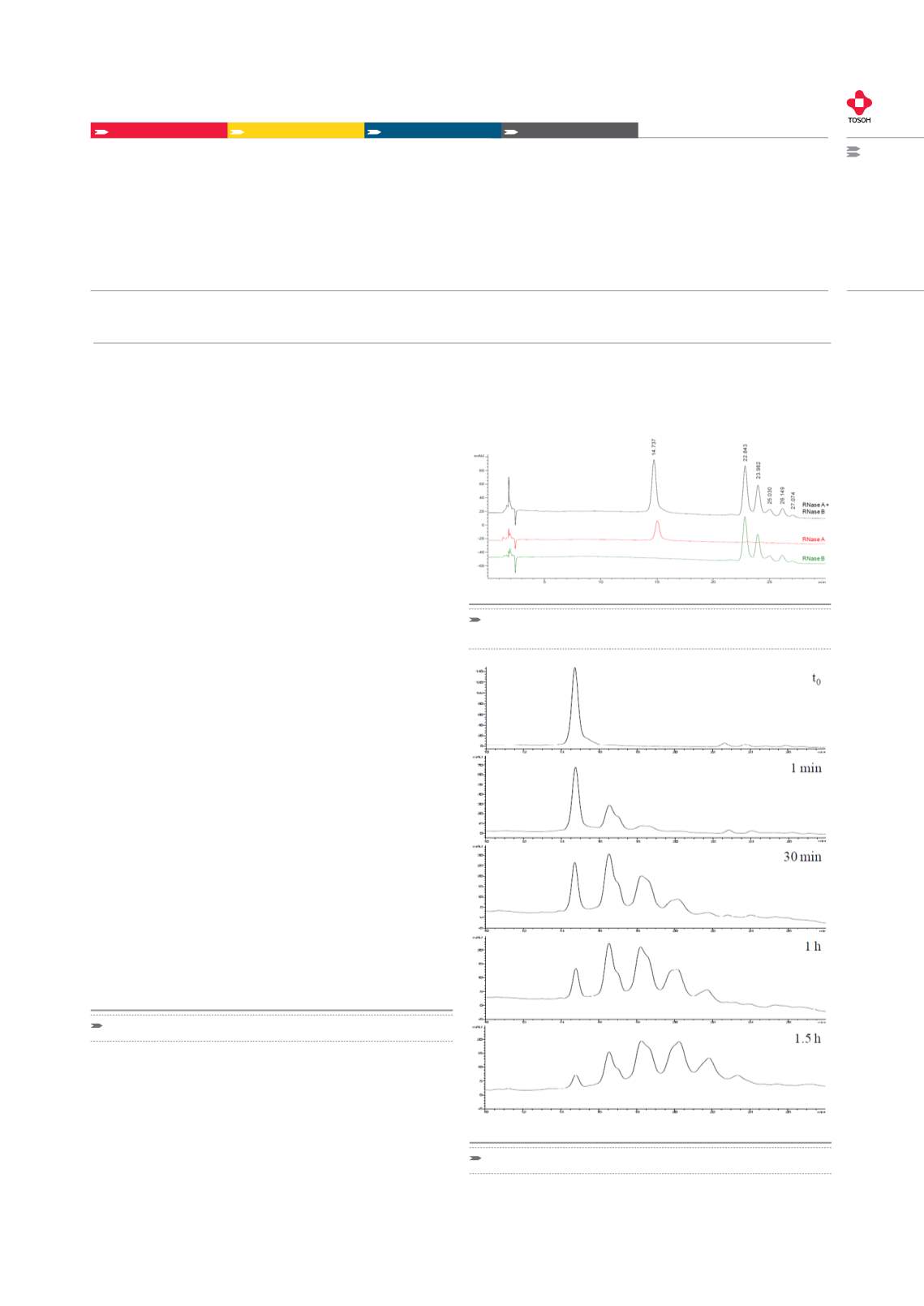
Figure 1 shows the chromatographic profiles for RNase A (red traces),
RNase B (green traces) and their equimolar mixture (black traces) on
TSKgel Amide-80 (2 x 150 mm, 3µm), eluted at a flow rate of 0.2 mL/
min and a temperature of 50 °C.
The method was applied to the separation of neo-glycoproteins pre-
pared starting from the RNase A by chemical conjugation of different
glycans. The presence of RNase A and its glycosylated reaction pro-
ducts were further confirmed by ESI-MS analysis. Applying the deve-
loped HILIC-UV method it was possible to monitor the glycosylation
reaction of RNaSe A with Ara (1-6)Man-IME and assess the distribu-
tion of neo-glycoprotein isoforms without laborious sample workup
prior to analysis (Figure 2).
HILIC applications in pharmaceutical research
Hydrophilic interaction liquid chromatography (HILIC) has gained an important role in pharmaceutical and biophar-
maceutical analysis. Herein we summarize one of the most recent papers from our featured lab, the department
of Drug Sciences, Pavia. Target molecules of their research range from cosmetics component over purine and py-
rimidine bases and nucleosides to intact Glycoproteins. Their work on the analysis of intact Neo-Glycoproteins by
HILIC was published in 2014 [1].
07
HILIC
In the Literature
[1]
Alice Pedrali et al., Molecules 2014, 19, 9070-9088
Figure 1:
HILIC Separation of RNase A and RNase B isoforms on
TSKgel Amide-80
Figure 2:
Monitoring of the synthesis of neo-glycoconjugates


